
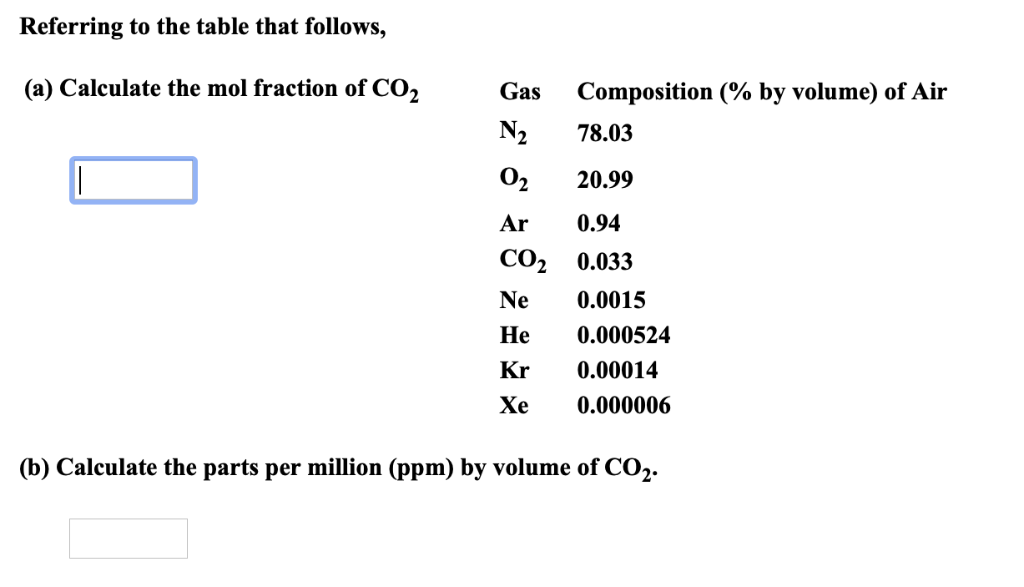
One thousanth of a gram is one milligram and 1000 ml is one liter, so that 1 ppm 1 mg per liter mg/Liter. When ppm is used as a measure for the suspended particle concentration, it is therefore very important to specify if the concentrations are ppm BY VOLUME or ppm BY MASS, to facilitate comparisons with data where the concentrations are reported in µl/l or mg/l.ĭo you have a need for an instrument that can measure particle concentration? Have a look at our LISST-Portable|XR laser for laboratory use, our LISST-200X laser for field and submersible use, or our LISST-AOBS that measures concentration using acoustics. 2 days ago &0183 &32 This calculation will allow you to dose to the 1. For example, a sample with a mass concentration of 100 mg/l will have a volume concentration of 38 µl/l. To convert from ppm by mass to ppm by volume, divide by the density of the particles. For example, a sample with a volume concentration of 25 µl/l will have a mass concentration of 25*2.65 = 66 mg/l. For example, 1 liter per second of CO2 in 100 l/s of exhaust would be 1 or 10,000 ppm. For mineral grains (clay, silt and sand sizes), this will typically be 2.65 g/cm3. Welcome to PF The steady state dilution equation is just the ratio of the contaminant entering to the total volume leaving the room, in any units as long as they are consistent. To convert from ppm by volume to ppm by mass, multiply by the density of the particles. However, if ppm is expressed as THE MASS of particles in a unit volume of water, then ppm BY MASS is equal to mg/l. 1 gallon 137,381 Btu (for distillate fuel with 15 ppm or less sulfur content). Also, use P ercent to PPM Calculator for more conversion. Example: find how many percent are in 300ppm. If ppm is expressed as THE VOLUME of particles to a unit volume of water, then ppm BY VOLUME is equal to µl/l. So to convert from ppm to percent, divide the ppm by 10000: x () x (ppm) / 10000.
To convert ppm you x by 104 to give 300ppm. E.g a gas in the atmosphere could be 0.03. But what parts? The amount of particles in a suspension can be expressed as the total volume OR total mass of particles in a unit volume of water AND THESE TWO NUMBERS WILL ONLY BE THE SAME IF THE DENSITY OF THE PARTICLES IS 1 g/cm3. Just as a percentage is 1 part in 100 ppm is one part in a million.


 0 kommentar(er)
0 kommentar(er)
